During an electrolysis of molten sodium chloride a 4A current is passed through electrodes for 1 hour. The caustic soda solution may be clear or slightly cloudy in appearance depending on the cell process used.

What Is Electrolysis Electrolysis Of Molten Sodium Chloride
As sodium violently reacts with air it is not found freely in nature.

. Sodium metal that forms at the cathode floats up through the molten sodium chloride into a sodium-collecting ring from which it is periodically drained. Given I 4A t 1 x 60 x. 2Cl- Cl 2 2e- 2H 2 O 2e- H 2 20H-2H 2 0 O 2 4H 4e-Subsequently chlorine and hydroxide react to form hypochlorite.
Rosenmund Reduction Acyl chloride acid chloride is hydrogenated over catalyst palladium on barium sulphate. The atomic structure or arrangement of these electrons is shown in the figure below. Chlorine and hydrogen are co-produced.
OxyChem produces sodium hydroxide NaOH solution commonly known as caustic soda through the electrolysis of sodium chloride salt brine in diaphragm or membrane cells. Hydrogen is used as a fuel and for making ammonia. It readily absorbs carbon.
If potassium chloride or calcium chloride is used instead potassium hydroxide and calcium hydroxide are produced. When one chemical reacts to a stimulus by breaking into two simpler elements AB into A and. Distilled water will not conduct current while tap water will conduct a small current.
The chloralkali process is a process used to create sodium hydroxide chlorine and hydrogen from sodium chloride and waterIt is normally conducted in a tank separated by a membrane although there are other methods such as using mercury. Combustion occurs when a chemical combines with oxygen. Chlorine can be manufactured by the electrolysis of a sodium chloride solution which is known as the Chloralkali process.
The electrolyte of alkaline water electrolysis systems is an aqueous solution of potassium or sodium hydroxide. 4NaCl- 4Na 4Cl-By leading the salty solution over an electrolysis cell the following reactions take place at the electrodes. Sodium Na is a highly reactive silvery metal.
The chemical change is one in which the substance loses or gains an electron oxidation or reduction. The electrolysis of brine is a large-scale process used to manufacture chlorine from salt. The solution with baking soda will facilitate a good amount of electrolysis.
Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Two other useful chemicals are obtained during the process sodium hydroxide NaOH and hydrogen H 2. The solution with table salt will facilitate electrolysis the best.
The potassium or sodium hydroxide concentrations which can vary as a function of the working temperature is generally in the 25 wt to 30 wt range for temperatures between 70C and 100C and pressures between 1 bar and 30 bars 145. Calculate the mass of sodium that is produced during this time. The process is carried out in an electrolytic cell an apparatus consisting of positive and negative electrodes held apart and dipped into a solution containing positively and.
Sodium chloride --- sodium chloride table salt Combustion reactions are often included in lists of synthetic reactions. These two products as well as chlorine itself are highly reactive. As you can see above the Na atom left side of.
Baking soda known by chemists as sodium bicarbonate NaHCO3 isnt an electrolyte on its own. The product is usually fire or another type of heat. Brine is a solution of sodium chloride NaCl and water H 2 O.
The production of chlorine results in the co-products caustic soda sodium hydroxide NaOH and hydrogen gas H 2. The atomic number of Na and Cl are 11 and 17 respectively. The diaphragm that separates the two electrodes is a screen of iron gauze which prevents the explosive reaction that would occur if the products of the electrolysis reaction came in contact.
That means the sodium atom has 11 electrons and the chlorine atom has 17 electrons orbiting them. Chlorine is used. Examples of Decomposition Chemical Reactions.
Of the electrolysis of concentrated sodium chloride solution have important uses in the chemical industry. For example in the electrolysis of molten sodium chloride sodium chloride is melted above 801 o C two electrodes are inserted into the melt and an electric current is passed through the molten salt. Na e.
With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. The chemical reaction that takes place at the electrodes are. This is known as Kolbes reaction.
This reaction is called Rosenmund reduction. The process of electrolysis involves using an electric current to bring about a chemical change and make new chemicals. Sodium-ion migrates to the cathode where sodium ion gains one electron and reduce to sodium metal.
It is obtained from dry fused sodium chloride through electrolysis. Phenol with sodium hydroxide gives sodium phenoxide ion which with carbon dioxide in acidic medium results hydroxybenzoic acid salicylic acid. Sodium Na and chloride Cl- ions are produced.
Sodium chloride is the common salt we use in our day to day life. It belongs to the alkali group of metals and. Sodium chloride is the salt.
Electrolysis process by which electric current is passed through a substance to effect a chemical change. OH- Cl 2 HOCl Cl-The advantage of the salt electrolysis. Sodium is abundantly found on the earth and also occurs in minerals like cryolite zeolite and amphibole.

Electrolysis Of Molten Sodium Chloride Teaching Chemistry Chemistry Classroom Chemistry Education

Electrolysis Of Sodium Chloride Membrane Chemistry Solutions

Bbc Gcse Bitesize Electrolysis Gcse Chemistry Science Revision Science
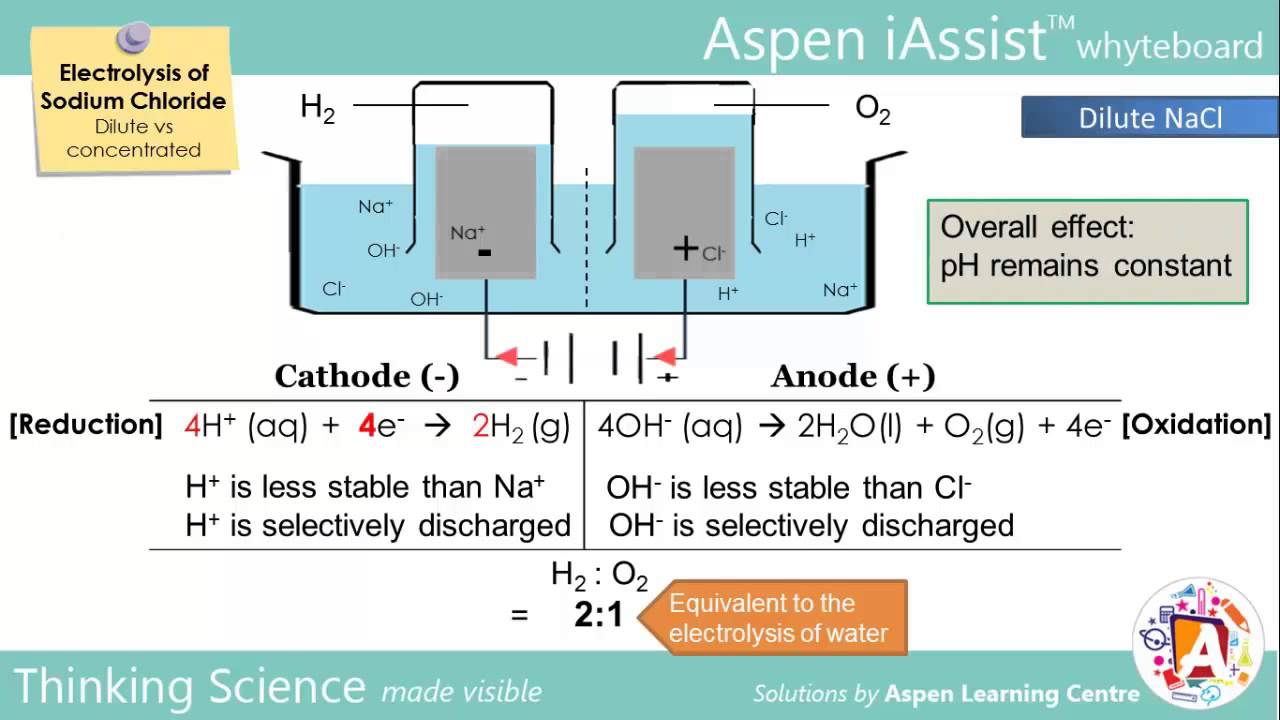
Electrolysis Of Nacl Dilute Vs Concentrated Free Ebooks Ebook Chemistry
0 Comments